Disease Background
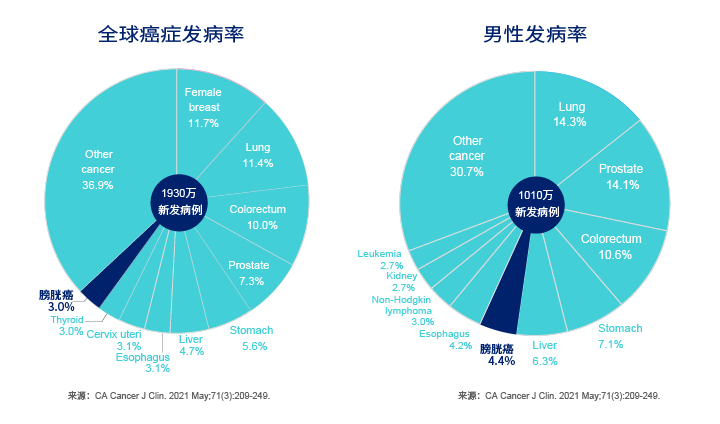
- High incidence:Bladder cancer is the most prevalent tumor of the urinary system. The number of new cases is more than 80,000 and the number of deaths is more than 30,000.
- High mortality: MRI index of Men’s bladder cancer is 0.40 (mortality/incidence) ,which higher than other tumor.
- High recurrence rate: The 5-year recurrence rate of non-muscular invasive bladder cancer (NMIBC) is as high as 60-78%; the recurrence rate of upper urinary tract urothelial carcinoma (UTUC) is 22%-47%[1].
- High treatment cost: The cost of treating bladder cancer by medical insurance ranks top 4 cancers[2].

- Ultrasound imaging: Sensitivity 63-98%, dependence on physician experience, and inability to diagnose carcinoma in situ.
- Urinary tumor marker: Low specificity, and susceptibility to hematuria interference.
- Urine cytology: Sensitivity 13-75%, difficulty in detecting LG-UC, and inability to exclude diagnosis based on a negative result.
- Fluorescence in situ hybridization (FISH): Low throughput, complex operation, and dependence on pathologist experience.
- Cystoscopy + tissue biopsy: Liability to overlooking carcinoma in situ, invasive examination, and poor compliance of the patients of postoperative review.
Product Advantages


-
87.4%Sensitivity
-
91.5%Specificity
-
89.3%Accuracy
-
Accurate performance
Accuracy 89.3%, equivalent to gold standard: cystoscopy.
-
Painless and convenient
Just 100ml of random urine needs to be collected for testing. It is more comfortable.
-
Proprietary patent
The detection method was granted with a Chinese Invention Patent (patent No. ZL2019 1 1370095.5).
-
Professional recognition
Verification and use experience of large clinical studies in hundreds of grade-A tertiary hospitals. The detection method and performance were published on professional medical journals The Journal of Clinical Investigation and Clinical Epigenetics.
It has been recognized at home and abroad
-
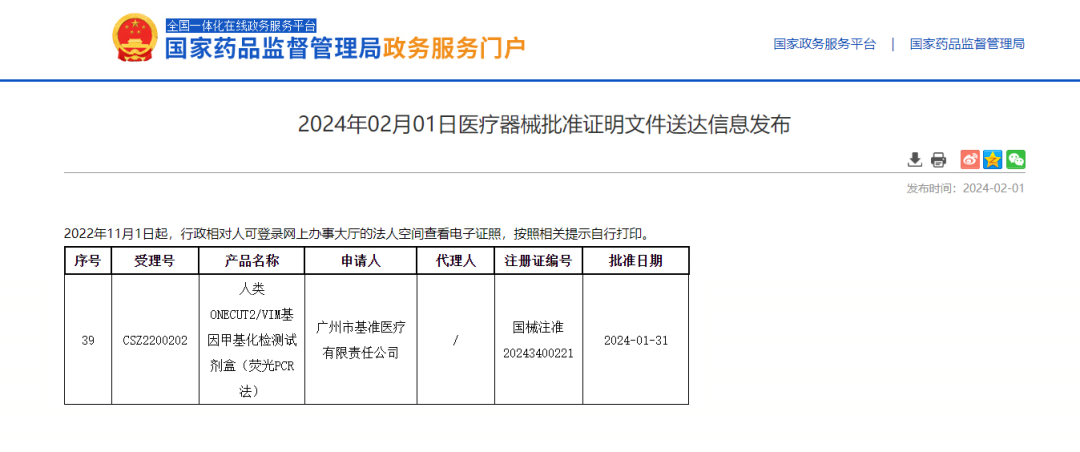
UriFind®Has Been Approved
China's First Urothelial Cancer Auxiliary Diagnostic Kit (UriFind) Has Been Approved and Launched on the Market
-
FDA certification
UriFind® earns "Breakthrough Device Designation" (BTD) from was identified as a breakthrough device (BTD) by U.S. Food and Drug Administration The breakthrough technology has significant advantages and is in the best interest of patients compared with the existing products.
-
CE certification
UriFind® obtained European Access Permit for in-vitro diagnostics devices (IVDD) authorized by the Netherlands CIBG
-
Inclusion in Guidelines
UriFindTMwas included in the Chinese Guidelines for Diagnosis and Treatment of Urological and Andrological Diseases (2022)
-
Awards & HonorsFirst Prize for Science and Technology Progress in Guangdong Province in 2021


-
Awards & HonorsGuangdong Famous High-Tech Product


Target Users
-

It is suitable for the auxiliary diagnosis of the patients who have hematuria and/or bladder irritation, or are advised by doctor to undertake cystoscopy.
Sample Collection and Service Process
-
100ml of urine
-
 Test counseling
Test counseling -
 Sample Collection
Sample Collection -
 Sample Transport
Sample Transport -
 Testing and Analysis
Testing and Analysis -
 Report issuance
Report issuance -
 After-Sales Service
After-Sales Service
References
- [1] Guidelines for Diagnosis and Treatment of Bladder Cancer (2018). GWBYH [2018] 1125.
- [2] China Statistical Yearbook of Health and Family Planning (2020).
- [3] J Clin Invest. 2020 Dec 1;130(12):6278-6289.
- [4] Clin Epigenetics. 2021 Apr 26;13(1):91.